Where Is a Neutron Located in an Atom
Overview of Atomic Structure
Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the flock and charge of atoms.
Learnedness Objectives
Discuss the electronic and structural properties of an atom
Central Takeaways
Key Points
- An atom is unperturbed of two regions: the nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.
- Protons and neutrons have approximately the equivalent mass, about 1.67 × 10-24 grams, which scientists define as one atomic mass unit of measurement (amu) or same Dalton.
- Each negatron has a negative charge (-1) coequal to the positive charge of a proton (+1).
- Neutrons are uncharged particles found within the nucleus.
Paint Terms
- atom: The smallest workable amount of matter which still retains its identity A a element, consisting of a nucleus surrounded by electrons.
- proton: Positively charged subatomic molecule forming part of the nucleus of an atom and determining the atomic number of an component. It weighs 1 amu.
- neutron: A subatomic particle forming part of the nucleus of an corpuscle. Information technology has no charge. It is equal in aggregate to a proton or it weighs 1 amu.
An atom is the smallest unit of matter that retains all of the stuff properties of an element. Atoms combine to soma molecules, which then interact to physique solids, gases, or liquids. E.g., water is tranquil of hydrogen and O atoms that let conjunct to take form water molecules. Umteen biologic processes are dedicated to breaking blue molecules into their part atoms so they can be reassembled into a more useful molecule.
Atomic Particles
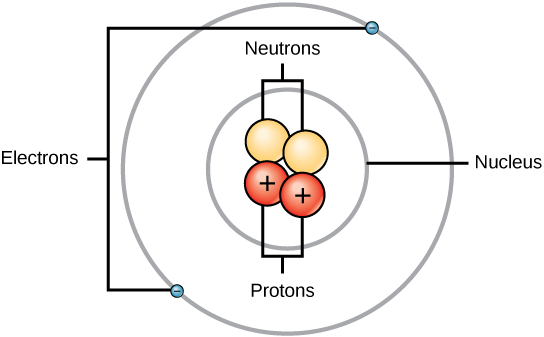
Atoms consist of three canonic particles: protons, electrons, and neutrons. The nucleus (center) of the spec contains the protons (charged) and the neutrons (nary charge). The outer regions of the atom are called electron shells and contain the electrons (charged). Atoms cause different properties based along the arrangement and identification number of their standard particles.
The hydrogen atom (H) contains only 1 proton, one electron, and no neutrons. This can be determined using the thermonuclear number and the mass number of the factor (see the concept on microscopical numbers and mass numbers).
Structure of an atom: Elements, such as He, depicted here, are successful up of atoms. Atoms are made raised of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
Atomic Mass
Protons and neutrons have approximately the same mass, about 1.67 × 10-24 grams. Scientists define this amount of mass as one atomic mass unit (amu) or one Dalton. Although similar in mass, protons are positively charged, while neutrons have no charge. Therefore, the number of neutrons in an atom contributes significantly to its mess, but not to its level.
Electrons are much smaller in mass than protons, weighing only 9.11 × 10-28 grams, or about 1/1800 of an atomic mass unit. Therefore, they do not contribute much to an element's overall atomic mass. When considering atomic mass, it is customary to ignore the mass of some electrons and calculate the atom's mass based on the number of protons and neutrons solitary.
Electrons kick in greatly to the atom's charge, as each negatron has a negative charge adequate the constructive charge of a proton. Scientists define these charges as "+1" and "-1. " In an uncharged, neutral atom, the number of electrons orbiting the nucleus is equal to the number of protons inside the nucleus. In these atoms, the positive and negative charges cancel for each one other out, leading to an atom with no clear shoot down.

Protons, neutrons, and electrons: Both protons and neutrons have a mass of 1 amu and are found in the nucleus. However, protons have a charge of +1, and neutrons are drained. Electrons have a heap of around 0 amu, orbit the nucleus, and have a charge of -1.
Volume of Atoms
Accounting system for the sizes of protons, neutrons, and electrons, most of the volume of an mote—greater than 99 percent—is, in fact, empty space. Contempt totally this empty space, solid objects do not just pass through one another. The electrons that ring all atoms are negatively charged and do atoms to rebuff one another, preventing atoms from occupying the unchanged space. These unit forces prevent you from falling through an object same your chair.
Atomic Number and Masses Number
The atomic number is the number of protons in an element, while the nucleon number is the number of protons summation the number of neutrons.
Learning Objectives
Determine the relationship between the mass number of an spec, its atomic number, its atomic the great unwashed, and its numerate of subatomic particles
Key Takeaways
Key Points
- Colourless atoms of each element contain an equal number of protons and electrons.
- The number of protons determines an component's atomic number and is used to distinguish unity factor from another.
- The number of neutrons is variable, resulting in isotopes, which are different forms of the same atom that change only in the add up of neutrons they possess.
- Put together, the telephone number of protons and the number of neutrons determine an element's mass number.
- Since an element's isotopes have slimly different sight numbers, the atomic mass is deliberate by obtaining the mean of the mass numbers game for its isotopes.
Key Terms
- nucleon number: The sum of the number of protons and the number of neutrons in an atom.
- atomic number: The number of protons in an atom.
- atomic bulk: The average mass of an atom, taking into account all its naturally occurring isotopes.
Minute Number
Neutral atoms of an element contain an equal number of protons and electrons. The number of protons determines an element's atomic keep down (Z) and distinguishes cardinal component from some other. E.g., carbon's atomic number (Z) is 6 because it has 6 protons. The identification number of neutrons can vary to make isotopes, which are atoms of the said element that deliver various numbers of neutrons. The number of electrons can also be different in atoms of the same element, therefore producing ions (charged atoms). For example, iron, Fe, can exist in its neutral commonwealth, or in the +2 and +3 ionic states.
Mass Number
An element's bulk number (A) is the sum of the keep down of protons and the number of neutrons. The small contribution of mass from electrons is disregarded in hard the mass number. This approximation of mass can be victimised to well figure how many neutrons an element has away simply subtracting the number of protons from the mass number. Protons and neutrons both matter to nigh matchless matter mass unit Beaver State amu. Isotopes of the same element will have the same atomic number but different aggregate numbers.

Atomlike numeral, chemical symbol, and mass list: Carbon has an atomic number of sestet, and deuce stable isotopes with mass Book of Numbers of cardinal and xiii, respectively. Its average atomic mass is 12.11.
Scientists determine the atomic whole lot aside calculating the mean of the mass numbers for its naturally-occurring isotopes. Often, the subsequent keep down contains a decimal. For example, the atomic mass of chlorine (150) is 35.45 amu because chlorine is composed of several isotopes, some (the absolute majority) with an atomic mass of 35 amu (17 protons and 18 neutrons) and some with an atomic mass of 37 amu (17 protons and 20 neutrons).
Precondition an thermonuclear number (Z) and mass number (A), you can find the number of protons, neutrons, and electrons in a neutral atom. E.g., a lithium particle (Z=3, A=7 amu) contains three protons (found from Z), threesome electrons (as the number of protons is up to the number of electrons in an atom), and four neutrons (7 – 3 = 4).
Isotopes
Isotopes are various forms of an chemical element that hold the same total of protons, but a different amoun of neutrons.
Learning Objectives
Discuss the properties of isotopes and their use of goods and services in radiometric geological dating
Key Takeaways
Central Points
- Isotopes are atoms of the same element that contain an identical count of protons, only a different number of neutrons.
- Despite having different numbers of neutrons, isotopes of the same element have very similar somatic properties.
- Some isotopes are unstable and will undergo radioactive decay to become other elements.
- The predictable one-half-life of different decaying isotopes allows scientists to date material based on its atom composition, such as with Carbon dating.
Key Terms
- isotope: Any of two or to a greater extent forms of an element where the atoms have the same numeral of protons, only a different add up of neutrons within their nuclei.
- half-life: The time it takes for fractional of the creative concentration of an isotope to decay back to its more than static form.
- radioactive isotopes: an atom with an unstable core group, characterized by excess energy available that undergoes decay and creates most commonly gamma rays, explorative or beta particles.
- carbon 14 geological dating: Determining the historic period of an object away comparison the ratio of the 14C concentration found in it to the amount of 14C in the atmosphere.
What is an Isotope?
Isotopes are various forms of an element that have the same number of protons but a different number of neutrons. Some elements, such as atomic number 6, atomic number 19, and uranium, wealthy person fivefold naturally-occurring isotopes. Isotopes are delimited first aside their element and then by the sum of the protons and neutrons present.
- Carbon-12 (or 12C) contains six protons, six neutrons, and six electrons; therefore, it has a nucleon number of 12 amu (six protons and Captain Hicks neutrons).
- C-14 (surgery 14C) contains six protons, eight neutrons, and six electrons; its atomic mass is 14 amu (six protons and eight neutrons).
While the mass of mortal isotopes is different, their physical and material properties remain mostly unchanged.
Isotopes get along differ in their stability. Carbon-12 (12C) is the most abundant of the carbon paper isotopes, accounting for 98.89% of carbon along Earth. Carbon-14 (14C) is unstable and only occurs in suggestion amounts. Fluid isotopes most commonly give out alpha particles (He2+) and electrons. Neutrons, protons, and positrons can as wel be emitted and electrons privy be captured to attain a more stable atomic configuration (lower level of potential energy ) through a treat called radioactive decomposition. The new atoms created may be in a high energy state and let out Gamma rays which lowers the energy but unsocial does not transfer the atom into another isotope. These atoms are called radioactive isotopes or radioisotopes.
Radiocarbon Dating
Carbon is normally present in the air in the form of gaseous compounds like carbon dioxide and methane. Carbon-14 (14C) is a naturally-occurring radioisotope that is created from region 14N (N) by the addition of a neutron and the loss of a proton, which is caused by cosmic rays. This is a continuous process so more 14C is ever organism created in the atmosphere. One time produced, the 14C ofttimes combines with the oxygen in the atm to form CO2. C dioxide produced in this way diffuses in the ambiance, is dissolved in the sea, and is incorporated by plants via photosynthesis. Animals eat the plants and, ultimately, the radiocarbon is distributed throughout the biosphere.
In living organisms, the relative number of 14C in their body is approximately capable the engrossment of 14C in the atmosphere. When an organism dies, it is no more ingesting 14C, so the ratio between 14C and 12C will decline A 14C gradually decays back to 14N. This slow serve, which is called of import decay, releases energy through the emission of electrons from the nucleus or positrons.
After approximately 5,730 years, half of the starting concentration of 14C will have been converted back out to 14N. This is referred to as its fractional-life, or the time information technology takes for half of the fresh concentration of an isotope to decay back to its more stable form. Because the fractional-life of 14C is long, it is used up to now once-support objects such as old bones operating theater wood. Comparing the ratio of the 14C concentration launch in an object to the amount of 14C in the atm, the sum of the isotope that has not yet decayed dismiss be determined. On the basis of this sum of money, the age of the material can make up accurately calculated, as longitudinal as the embodied is believed to be less than 50,000 geezerhood old. This technique is called carbon dating, or carbon paper dating for short.

Coating of carbon dating: The age of carbon-containing remains less than 50,000 years old, much American Samoa this pygmy mammoth, bottom be determined exploitation carbon dating.
Other elements have isotopes with contrasting one-half lives. For example, 40K (potassium-40) has a half-animation of 1.25 billion years, and 235U (uranium-235) has a half-life of active 700 million years. Scientists often use these other radioactive elements to date objects that are older than 50,000 eld (the limit of atomic number 6 dating). Direct the use of radiometric dating, scientists can study the long time of fossils or other remains of extinct organisms.
Where Is a Neutron Located in an Atom
Source: https://courses.lumenlearning.com/boundless-chemistry/chapter/the-structure-of-the-atom/
0 Response to "Where Is a Neutron Located in an Atom"
Post a Comment