What Are Developmental Studies Reading Scores Used for
A paper's "Methods" (or "Materials and Methods") department provides information on the study's design and participants. Ideally, it should be and so clear and detailed that other researchers can repeat the study without needing to contact the authors. Yous will need to examine this section to make up one's mind the study's strengths and limitations, which both touch how the report'due south results should exist interpreted.
Demographics
The "Methods" section commonly starts by providing data on the participants, such as age, sex, lifestyle, health condition, and method of recruitment. This data will assistance you determine how relevant the study is to you, your loved ones, or your clients.
Effigy iii: Case study protocol to compare two diets
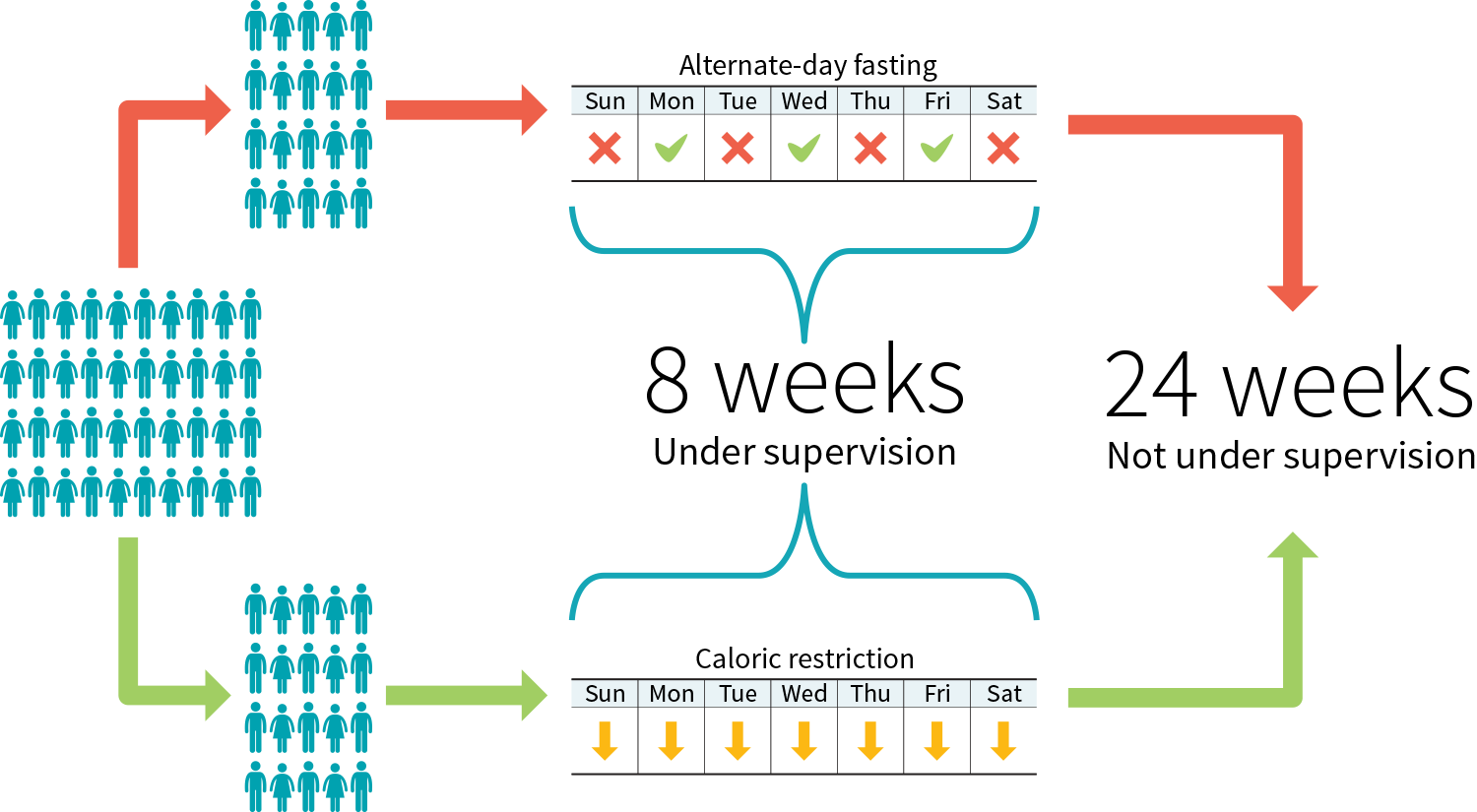
The demographic information can be lengthy, you might exist tempted to skip it, withal it affects both the reliability of the study and its applicability.
Reliability. The larger the sample size of a study (i.east., the more participants it has), the more reliable its results. Note that a study oft starts with more participants than it ends with; diet studies, notably, normally come across a fair number of dropouts.
Applicability. In health and fitness, applicability means that a compound or intervention (i.e., exercise, nutrition, supplement) that is useful for 1 person may be a waste of money — or worse, a danger — for some other. For instance, while creatine is widely recognized as condom and constructive, at that place are "nonresponders" for whom this supplement fails to better practise functioning.
Your mileage may vary, as the creatine example shows, yet a study'due south demographic information can assistance y'all appraise this study's applicability. If a trial only recruited men, for case, women reading the study should keep in heed that its results may be less applicative to them. Likewise, an intervention tested in college students may yield different results when performed on people from a retirement facility.
Figure four: Some trials are sexual practice-specific

Furthermore, different recruiting methods will attract different demographics, and and then can influence the applicability of a trial. In most scenarios, trialists will utilize some grade of "convenience sampling". For instance, studies run by universities will often recruit among their students. Nonetheless, some trialists volition use "random sampling" to make their trial's results more applicable to the general population. Such trials are generally called "augmented randomized controlled trials".
Confounders
Finally, the demographic information will unremarkably mention if people were excluded from the written report, and if then, for what reason. Most ofttimes, the reason is the beingness of a confounder — a variable that would confound (i.e., influence) the results.
For case, if y'all report the effect of a resistance training program on musculus mass, you don't want some of the participants to take muscle-building supplements while others don't. Either you'll want all of them to accept the same supplements or, more probable, you lot'll desire none of them to take whatever.
Likewise, if you lot study the issue of a muscle-building supplement on muscle mass, you don't want some of the participants to exercise while others do not. You'll either want all of them to follow the same conditioning program or, less likely, yous'll want none of them to practice.
It is of grade possible for studies to have more than two groups. Yous could accept, for instance, a study on the effect of a resistance training programme with the following iv groups:
-
Resistance preparation plan + no supplement
-
Resistance training program + creatine
-
No resistance training + no supplement
-
No resistance training + creatine
Merely if your written report has four groups instead of two, for each grouping to keep the same sample size yous demand twice as many participants — which makes your study more difficult and expensive to run.
When you come right downward to it, any differences between the participants are variable and thus potential confounders. That'south why trials in mice apply specimens that are genetically very close to one another. That's also why trials in humans seldom attempt to test an intervention on a diverse sample of people. A trial restricted to older women, for instance, has in effect eliminated age and sexual practice as confounders.
As nosotros saw higher up, with a peachy plenty sample size, we can have more groups. Nosotros tin even create more groups after the study has run its course, by performing a subgroup analysis. For instance, if you lot run an observational study on the effect of red meat on thousands of people, you can later separate the data for "male" from the information for "female" and run a separate analysis on each subset of data. Nonetheless, subgroup analyses of these sorts are considered exploratory rather than confirmatory and could potentially lead to false positives. (When, for instance, a blood exam erroneously detects a disease, information technology is called a fake positive.)
Design and endpoints
The "Methods" department volition likewise draw how the study was run. Design variants include single-blind trials, in which only the participants don't know if they're receiving a placebo; observational studies, in which researchers only observe a demographic and take measurements; and many more. (Meet effigy two above for more examples.)
More specifically, this is where y'all will acquire about the length of the study, the dosages used, the conditioning regimen, the testing methods, and so on. Ideally, equally we said, this data should exist so clear and detailed that other researchers can repeat the written report without needing to contact the authors.
Finally, the "Methods" section tin can besides make clear the endpoints the researchers volition be looking at. For example, a study on the effects of a resistance training program could use muscle mass equally its primary endpoint (its principal benchmark to judge the issue of the written report) and fat mass, forcefulness performance, and testosterone levels as secondary endpoints.
1 trick of studies that want to find an event (sometimes and then that they can serve equally marketing material for a product, but often only because studies that show an effect are more likely to become published) is to collect many endpoints, then to make the paper almost the endpoints that showed an consequence, either by downplaying the other endpoints or by not mentioning them at all. To prevent such "data dredging/angling" (a method whose devious efficacy was demonstrated through the hilarious chocolate hoax), many scientists push for the preregistration of studies.
Sniffing out the tricks used by the less scrupulous authors is, alas, part of the skills you'll need to develop to assess published studies.
Interpreting the statistics
The "Methods" section usually concludes with a hearty statistics discussion. Determining whether an appropriate statistical assay was used for a given trial is an entire subject, so we advise you don't sweat the details; endeavor to focus on the big picture.
First, let's clear up 2 mutual misunderstandings. Yous may have read that an result was significant, just to later discover that it was very small. Similarly, you lot may take read that no effect was plant, yet when y'all read the paper you found that the intervention grouping had lost more than weight than the placebo group. What gives?
The trouble is simple: those quirky scientists don't speak similar normal people do.
For scientists, significant doesn't mean important — it means statistically meaning. An effect is meaning if the data collected over the course of the trial would exist unlikely if at that place really was no effect.
Therefore, an issue can be significant yet very small — 0.ii kg (0.5 lb) of weight loss over a year, for instance. More than to the point, an consequence tin can exist pregnant nevertheless not clinically relevant (meaning that it has no discernible event on your wellness).
Relatedly, for scientists, no issue usually ways no statistically significant effect. That'south why you may review the measurements nerveless over the course of a trial and notice an increase or a decrease yet read in the determination that no changes (or no effects) were establish. There were changes, only they weren't significant. In other words, there were changes, but so small that they may exist due to random fluctuations (they may also be due to an actual effect; we can't know for certain).
We saw before, in the "Demographics" section, that the larger the sample size of a written report, the more reliable its results. Relatedly, the larger the sample size of a study, the greater its ability to discover if small effects are significant. A modest change is less likely to be due to random fluctuations when found in a written report with a m people, let'southward say, than in a written report with 10 people.
This explains why a meta-analysis may find significant changes by pooling the data of several studies which, independently, institute no significant changes.
P-values 101
Most ofttimes, an effect is said to be pregnant if the statistical analysis (run by the researchers post-study) delivers a p-value that isn't higher than a certain threshold (set up past the researchers pre-study). We'll telephone call this threshold the threshold of significance.
Understanding how to interpret p-values correctly tin can be tricky, even for specialists, but here's an intuitive manner to think about them:
Think most a coin toss. Flip a money 100 times and you volition get roughly a 50/50 split of heads and tails. Not terribly surprising. Just what if you flip this coin 100 times and get heads every time? At present that's surprising! For the record, the probability of information technology really happening is 0.00000000000000000000000000008%.
You can think of p-values in terms of getting all heads when flipping a coin.
-
A p-value of five% (p = 0.05) is no more surprising than getting all heads on 4 coin tosses.
-
A p-value of 0.five% (p = 0.005) is no more surprising than getting all heads on 8 money tosses.
-
A p-value of 0.05% (p = 0.0005) is no more surprising than getting all heads on 11 coin tosses.
Contrary to popular belief, the "p" in "p-value" does not represent "probability". The probability of getting 4 heads in a row is 6.25%, not 5%. If you want to catechumen a p-value into coin tosses (technically called S-values) and a probability pct, check out the converter here.
As we saw, an effect is pregnant if the data collected over the course of the trial would be unlikely if there really was no effect. Now we can add that, the lower the p-value (under the threshold of significance), the more than confident we can be that an issue is significant.
P-values 201
All right. Fair warning: we're going to get nerdy. Well, nerdier. Feel free to skip this department and resume reading here.
Nevertheless with u.s.? All right, then — let's get at it. Equally we've seen, researchers run statistical analyses on the results of their written report (usually one analysis per endpoint) in order to decide whether or not the intervention had an consequence. They commonly brand this decision based on the p-value of the results, which tells you how probable a result at least as farthermost as the one observed would exist if the null hypothesis, among other assumptions, were true.
Ah, jargon! Don't panic, we'll explain and illustrate those concepts.
In every experiment at that place are generally two opposing statements: the null hypothesis and the culling hypothesis. Allow's imagine a fictional study testing the weight-loss supplement "Better Weight" against a placebo. The two opposing statements would expect like this:
-
Null hypothesis: compared to placebo, Better Weight does not increment or subtract weight. (The hypothesis is that the supplement'south effect on weight is zero.)
-
Alternative hypothesis: compared to placebo, Better Weight does decrease or increment weight. (The hypothesis is that the supplement has an effect, positive or negative, on weight.)
The purpose is to see whether the effect (here, on weight) of the intervention (here, a supplement called "Better Weight") is improve, worse, or the same as the effect of the control (hither, a placebo, merely sometimes the control is some other, well-studied intervention; for case, a new drug tin can be studied against a reference drug).
For that purpose, the researchers ordinarily set a threshold of significance (α) before the trial. If, at the end of the trial, the p-value (p) from the results is less than or equal to this threshold (p ≤ α), there is a significant deviation between the effects of the ii treatments studied. (Remember that, in this context, significant ways statistically significant.)
Figure 5: Threshold for statistical significance

The most unremarkably used threshold of significance is 5% (α = 0.05). It means that if the null hypothesis (i.e., the idea that there was no difference between treatments) is true, then, after repeating the experiment an space number of times, the researchers would become a faux positive (i.e., would notice a significant effect where there is none) at nearly 5% of the fourth dimension (p ≤ 0.05).
Generally, the p-value is a measure of consistency between the results of the study and the idea that the 2 treatments take the same effect. Let'south encounter how this would play out in our Better Weight weight-loss trial, where i of the treatments is a supplement and the other a placebo:
-
Scenario 1: The p-value is 0.lxxx (p = 0.80). The results are more consistent with the null hypothesis (i.e., the thought that there is no difference between the ii treatments). We conclude that Meliorate Weight had no significant outcome on weight loss compared to placebo.
-
Scenario 2: The p-value is 0.01 (p = 0.01). The results are more consistent with the alternative hypothesis (i.e., the thought that there is a difference betwixt the two treatments). We conclude that Meliorate Weight had a significant effect on weight loss compared to placebo.
While p = 0.01 is a meaning event, and so is p = 0.000001. So what information practice smaller p-values offer us? All other things being equal, they give us greater conviction in the findings. In our instance, a p-value of 0.000001 would give us greater confidence that Better Weight had a significant effect on weight alter. But sometimes things aren't equal between the experiments, making direct comparing between ii experiment's p-values catchy and sometimes downright invalid.
Even if a p-value is meaning, recollect that a significant effect may not be clinically relevant. Allow'due south say that we found a significant result of p = 0.01 showing that Meliorate Weight improves weight loss. The catch: Better Weight produced but 0.2 kg (0.5 lb) more weight loss compared to placebo subsequently 1 year — a difference as well pocket-sized to accept any meaningful effect on health. In this case, though the issue is significant, statistically, the existent-globe result is too small to justify taking this supplement. (This blazon of scenario is more than likely to take identify when the written report is big since, as nosotros saw, the larger the sample size of a study, the greater its power to observe if small effects are significant.)
Finally, we should mention that, though the nigh commonly used threshold of significance is 5% (p ≤ 0.05), some studies require greater certainty. For example, for genetic epidemiologists to declare that a genetic association is statistically significant (say, to declare that a gene is associated with weight gain), the threshold of significance is usually set at 0.0000005% (p ≤ 0.000000005), which corresponds to getting all heads on 28 money tosses. The probability of this happening is 0.00000003%.
P-values: Don't worship them!
Finally, keep in mind that, while important, p-values aren't the final say on whether a study'due south conclusions are authentic.
We saw that researchers too eager to detect an upshot in their report may resort to "data line-fishing". They may also endeavor to lower p-values in diverse ways: for instance, they may run unlike analyses on the same information and only study the significant p-values, or they may recruit more and more participants until they get a statistically significant result. These bad scientific practices are known as "p-hacking" or "selective reporting". (You can read about a existent-life instance of this here.)
While a written report's statistical analysis usually accounts for the variables the researchers were trying to command for, p-values can also be influenced (on purpose or non) by study design, hidden confounders, the types of statistical tests used, and much, much more. When evaluating the force of a written report's design, imagine yourself in the researcher's shoes and consider how y'all could torture a study to make it say what you desire and advance your career in the process.
Source: https://examine.com/guides/how-to-read-a-study/
0 Response to "What Are Developmental Studies Reading Scores Used for"
Post a Comment